BACKGROUND
The combination of venetoclax (VEN) and azacitidine (AZA) is the actual standard of care for patients with acute myeloid leukemia (AML) not eligible for intensive treatment ( Di Nardo et al. N Engl J Med 2020; 383:617-629). Despite being considered a low-intensity treatment, dose adjustments are frequently needed in this group of patients due to prolonged myelosuppression and increased incidence of infection. Reducing the duration of VEN administration might improve the safety profile, although its efficacy is still unknown in this setting.
AIM
We present the experience of the Institut Català d'Oncologia-Hospital Duran i Reynals in patients with AML treated with VEN in combination with hypomethylating agents (HMA) to evaluate the efficacy of VEN exposure reduction in patients who require VEN adjustment due to myelotoxicity.
METHODS
We retrospectively analyzed 68 patients with AML treated with VEN+HMA as a first-line or as salvage therapy between May 2019 and April 2023. We specifically evaluated patients in complete remission (CR) after 1 to 3 cycles of VEN combination who require VEN scheme shortening to 7 (low dose) or 14-21 (intermediate dose) days every 28 days cycle due to myelotoxicity. Response to treatment was defined according to ELN2022 criteria.
RESULTS
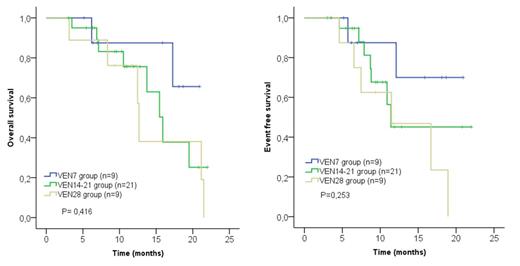
Median age at diagnosis was 75 years (range 33-85 years). Forty-seven (69%) were male. Twenty-eight (41.2%) were classified according to the 2022 ELN classification as an adverse risk, 33 (48.5%) as intermediate risk and 7 (10.3%) as a favorable risk group. The median number of cycles was 7 (range 1-23). ORR of the whole series was 76.5%. Forty-six (67.6%) patients achieved a composite complete response (CRc) (CR, CR with partial hematologic recovery, and CR with incomplete hematologic recovery). Among the 46 responding patients, seven of them (15.2%) discontinued treatment because of patient's decision after achieving CR and they were not considered for further analysis. The remaining 39 patients reached the best response in the first 2 cycles. Twenty-eight (71.8%) patients received VEN+HMA as first-line treatment and 11 (28.2%), as salvage therapy. No significant differences were found between these two groups regarding sex, age or ELN2022 risk. Nine out of these 39 patients (23.1%) received VEN for 28 days every 28 days cycle (VEN28 group), 21 (53.8%) patients received VEN for 14 or 21 per cycle (VEN14-21 group), and 9 (23.1%) patients received VEN for 7 days per cycle (VEN7 group). With a median follow-up of 18.8 months (range 14.6-23.1), the median OS of the VEN14-21 group was 15.9 months but it was not reached for the VEN7 group. Regarding EFS, the median EFS of the VEN14-21 group was 11.4 months though it was not reached for the VEN7 group. No statistically significant differences were found comparing OS and EFS between the VEN14-21 group and the VEN7 group nor were both of them with the VEN28 group (Figure 1).
CONCLUSION
Shortening VEN exposure to 14-21 or 7 days per cycle with concomitant HMA administration seems to induce a similar response rate and is likely to have comparable efficacy to the 28-day cycle. Among responders, VEN dose reduction seems to be associated with favorable outcomes. Prospective clinical trials that further evaluate the efficacy and safety of shortened VEN administration are required.
Disclosures
Sureda Balari:Astra Zeneca: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Pierre Fabre: Consultancy, Honoraria; GenMab: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; MSD: Consultancy, Honoraria; Jannsen: Consultancy, Honoraria; Kite: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria.